Abstract
Background: Daratumumab is an anti-CD38 monoclonal antibody approved in combination with pomalidomide/dexamethasone (DPd), bortezomib/dexamethasone (DVd) and carfilzomib/dexamethasone (DKd) for relapsed/refractory multiple myeloma (RRMM). We conducted a single-center retrospective study to analyze the safety and efficacy of DPd versus (vs) DVd in daratumumab naïve RRMM patients.
Methods: We evaluated RRMM patients who had received either DPd or DVd for RRMM at the University of Kansas Cancer Center between January 2015 and June 2022. DPd was given as daratumumab 16 mg/kg IV or 1800 mg SQ weekly for cycles 1 and 2, every 2 weeks for cycles 3-6, and then every 4 weeks; pomalidomide was dosed at 4 mg orally on days 1-21 of a 28-day cycle; and dexamethasone 20 or 40 mg weekly. The DVd group received daratumumab 16 mg/kg IV or 1800 mg SQ weekly for 3 cycles, every 3 weeks for cycles 4-8, and then every 4 weeks afterward, bortezomib (1.3 mg/m2) day 1,4,8,11 every 3 weeks for 8 cycles; dexamethasone 20 or 40 mg weekly in combination with daratumumab. Responses were evaluated using IMWG criteria and toxicities were determined using common terminology criteria for adverse events (CTCAE) grading. Patient and disease characteristics, and safety and efficacy outcomes were summarized with descriptive statistics. Kaplan-Meier analyses were used to estimate progression-free (PFS) and overall survival (OS).
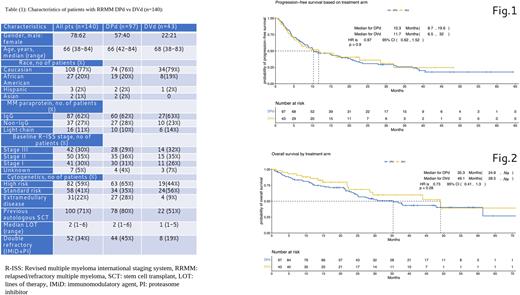
Results: A total of 140 pts with RRMM were included in the analysis; 97 patients received DPd, and 43 pts received DVd. The median age for the entire patient population was 66 years [range 38-84], 78 [56%] patients were male, 87 (62%) patients had IgG isotype, 42 (30%) R-ISS stage III disease, 82 (59%) patients had high-risk cytogenetics, and 31(22%) patients had extramedullary disease (EMD) (Table 1). The two groups appeared fairly balanced except for more patients in the DPd group had EMD, prior transplant and double refractory (refractory to proteasome inhibitors and immunomodulatory agents). A total of 15 patients who had bortezomib refractory disease received DVd while none of the patients with pomalidomide refractory disease received DPd. The overall response rate (ORR) and rate of patients who achieved a very good partial response was 71% (69) and 47% (46) of the DPd patients, vs 70% (30) and 28% (12) of the DVd patients respectively. With a median follow up of 37 months, median PFS was 10.3 months (95% CI 8.7-19.6) and 11.7 months (95% CI 6.5-32, p=0.9) for the DPd and DVd groups, respectively. (Figure.1) Median OS was 35.3 months (95% CI 24.8-Na) and 49.1 months (95% CI 28.5-Na, p= 0.28) for DPd and DVd groups, respectively. (Figure 1) The most common grade 3 or 4 adverse events were neutropenia (74% vs 14%), anemia (21% vs 9%), thrombocytopenia (12% vs 40%), and neuropathy (2% vs 23%), in the DPd group vs DVd group, respectively. Serious adverse events occurred in 27% in the DPd group vs 39% in the DVd group; with pneumonia being the most common serious adverse event (14 [14%] vs 9 [21%]). More pts in the DPd group (70%) required dose reductions due to toxicities than DVd group (51%).
Conclusion: Both DPd and DVd appear to be a safe and effective treatment options for RRMM. While there is more neutropenia associated with DPd and more neuropathy with DVd, there is no significant difference between the efficacy of the two regimens. Prospective, randomized studies are needed to confirm these findings.
Disclosures
Atrash:Celgene: Honoraria, Speakers Bureau; GSK: Honoraria, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Takeda: Honoraria; Amgen: Research Funding. Paul:Regeneron: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Amgen: Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees. Mohan:Takeda: Research Funding; GSK: Research Funding; BMS/Celgene: Research Funding; Novartis: Research Funding. Alkharabsheh:Amgen: Consultancy; National Community Oncology Dispensing Association, Inc: Consultancy; Incyte: Consultancy; AstraZeneca: Consultancy; Genentech: Consultancy; Agios: Consultancy. Mahmoudjafari:BMS: Membership on an entity's Board of Directors or advisory committees; Merk: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Omeros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Hashmi:JANSSEN: Consultancy; KARYOPHARM: Speakers Bureau; GSK: Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.